Group Brandau
Our Group
Our research group works on different projects concerning the immune system in cancer. Our research focuses on head and neck tumors, but in connection with various collaborators we also explore further tumor entities and diseases. For an optimal professional exchange, we work in global networks with several research groups.
Basic Research & Clinical Studies
The basis of our work contributes to basic research. However, in the context of our cooperative projects we also work on clinical studies. To do both basic research and clinical studies we use highly innovative methods.

Prof. Dr.
Sven Brandau
Head of Experimental & Translational Research
What we are proud of
A special success are our international COST Actions:
World Cancer Day 2026 – Transforming hidden immune-data into better healthcare
Mye-InfoBank 2021 – 2025 – Converting molecular profiles of myeloid cells into biomarkers for inflammation and cancer.
Mye-EUNITER 2014 – 2018 – European Network of Investigators Triggering Exploratory Research on Myeloid Regulatory Cells.
Those networks contribute to long-lasting connections, friendships and breakthroughs even beyond the duration of the funding. Through joint projects and symposia we were able to reach a common consensus on markers, protocols and analytical methods. The Mye-EUNITER network has developed uniform analytical methods for the investigation of MDSC immune cells (myeloid derived suppressor cells). The Mye-InfoBank network will exploit publicly available OMICS data sets with the aim to convert these into practical immune-based disease biomarker protocols and applications.
Links
World Cancer Day
Mye-InfoBank
Mye-EUNITER
The most important approaches of our scientific work:
Functional Immunology & Immunomonitoring
The control of immunological function is essential for the development of successful therapies.
We investigate the effect of therapeutics, the mechanisms of resistance to therapy and evaluate therapy response based on various biomarkers.
Murine Tumor Models, Imaging & Immunotherapy
The work with murine tumor models establishes the fundament for new and more effective therapeutic methods.
Especially for immunotherapy, numerous questions still need to be addressed before clinical implementation.
Research Themes and Projects
Our research comprises the following thematic and technological project areas.
Human neutrophils in malignant diseases
Neutrophil granulocytes are of central importance for the protection of our body against infections. For many years neutrophils have been regarded as terminally differentiated cells, which primarily phagocytose and neutralize infectious pathogens. More recently, the field is changing in several ways: First, neutrophils are not a homogenous population of cells but rather comprise distinct subsets. Second, these subsets are functionally specialized. Third, neutrophils exert surprising functional plasticity, which is regulated in a context- and tissue-specific manner.
In our projects we are analyzing the function of peripheral blood and tumor tissue-associated neutrophils from healthy donors and cancer patients. We have deciphered several mechanisms by which neutrophils, exert either anti-tumor or pro-tumor activity. Among those mechanisms is the induction of T-cell-mediated immunity in the context of cancer immunotherapy, the promotion of metastasis by modulation of cancer cell properties as well as a potential role in mediating the beneficial effects of extracorporeal photophoresis in the treatment of chronic graft-versus-host disease. It is our aim to better understand the increasing complexity of neutrophils in the tumor host in order to therapeutically modulate their biology.
Our research has triggered the release of several well-cited reviews, which will provide you with a comprehensive overview of the field.
Moses K, Brandau S. (2016) Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. 28 (2), 187-96.
https://doi.org/10.1016/j.smim.2016.03.018
Antuamwine BB, Bosnjakovic R, Hofmann-Vega F, Wang X, Theodosiou T, Iliopoulos I, Brandau S. N1 versus N2 and PMN-MDSC: A critical appraisal of current concepts on tumor-associated neutrophils and new directions for human oncology. Immunol Rev. 2023; 314(1):250-279
https://doi.org/10.1111/imr.13176
Monitoring, functional analysis and clinical relevance of myeloid-derived suppressor cells (MDSC)
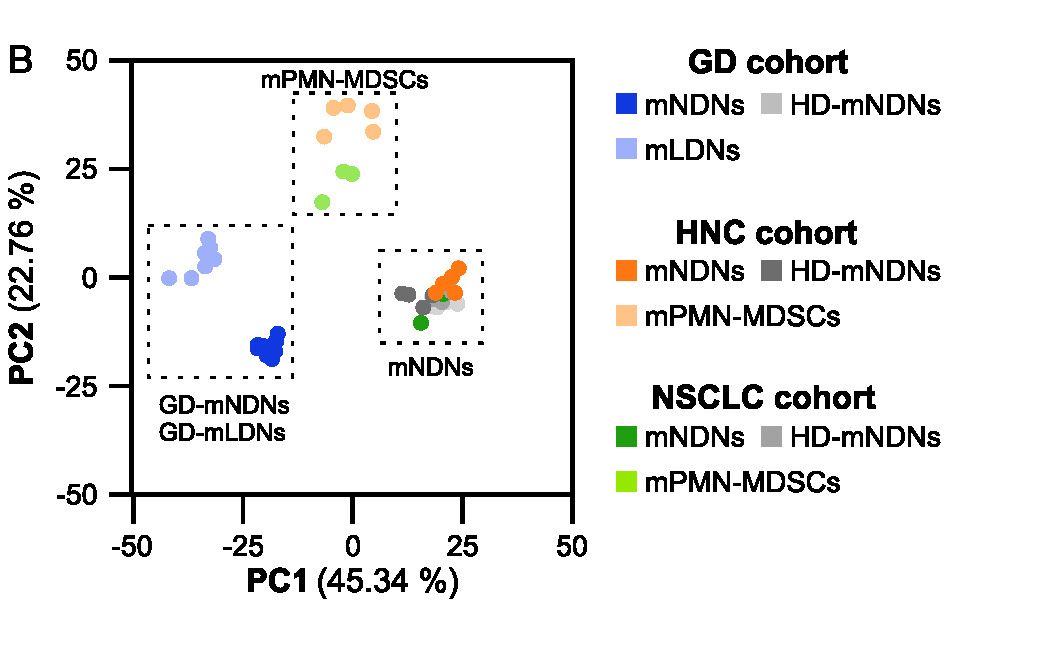
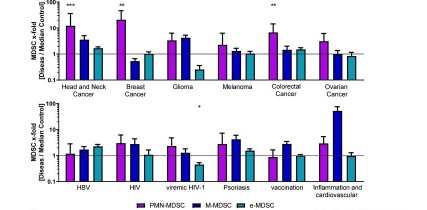
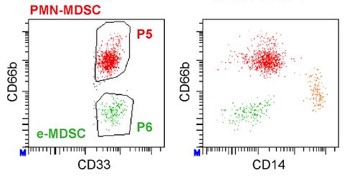
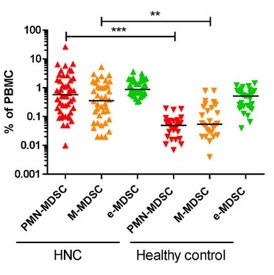
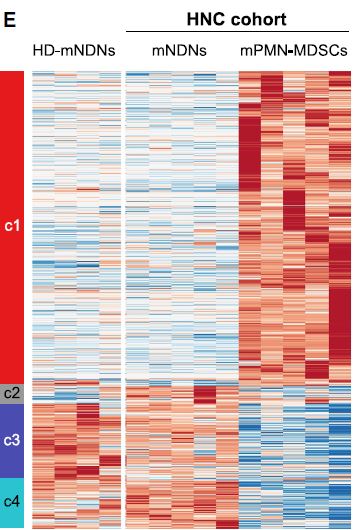
MDSC are a heterogeneous population of myeloid cells, which typically expand under pathological conditions. MDSC consist of myeloid cells of various differentiation and activation stages and comprise mononuclear (monocytic) and polymorphonuclear (granulocytic) subsets. The immunosuppressive activity on T cells and other types of immune cells is a hallmark of MDSC. Our lab is among the groups, who pioneered the field of human MDSC analysis, and we are currently involved in multiple international activities aimed at improving the analysis and potential therapeutic exploitation of these cells.
We are currently exploring the functional activities and clinical relevance of the three major human MDSC subsets in head and neck cancer and other solid tumors. We have also coordinated a pan-European study designed to compare MDSC phenotypes and frequencies in different malignant, infectious and inflammatory disease conditions. Reviews are available to provide more insight into the biology of these emerging myeloid regulatory cells as well as their relationship to neutrophils.
Pettinella F, Mariotti B, Lattanzi C, Bruderek K, Donini M, Costa S, Marini O, Iannoto G, Gasperini S, Caveggion E, Castellucci M, Calzetti F, Bianchetto-Aguilera F, Gardiman E, Giani M, Dusi S, Cantini M, Vassanelli A, Pavone D, Milella M, Pilotto S, Biondani P, Höing B, Schleupner MC, Hussain T, Hadaschik B, Kaspar C, Visco C, Tecchio C, Koenderman L, Bazzoni F, Tamassia N, Brandau S, Cassatella MA, Scapini P. (2024)
Surface CD52, CD84, and PTGER2 mark mature PMN-MDSCs from cancer patients and G-CSF-treated donors.
Cell Rep Med. 2024 Jan 17:101380
Cassetta L, Bruderek K, Skrzeczynska-Moncznik J, Osiecka O, Hu X, Rundgren IM, Lin A, Santegoets K, Horzum U, Godinho-Santos A, Zelinskyy G, Garcia-Tellez T, Bjelica S, Taciak B, Kittang AO, Höing B, Lang S, Dixon M, Müller V, Utikal JS, Karakoç D, Yilmaz KB, Górka E, Bodnar L, Anastasiou OE, Bourgeois C, Badura R, Kapinska-Mrowiecka M, Gotic M, Ter Laan M, Kers-Rebel E, Król M, Santibañez JF, Müller-Trutwin M, Dittmer U, de Sousa AE, Esendağlı G, Adema G, Loré K, Ersvær E, Umansky V, Pollard JW, Cichy J, Brandau S, (2020) Differential expansion of circulating human MDSC subsets in patients with cancer, infection and inflammation. J Immunother Cancer. 2020 Sep; 8(2): e001223
http://dx.doi.org/10.1136/jitc-2020-001223
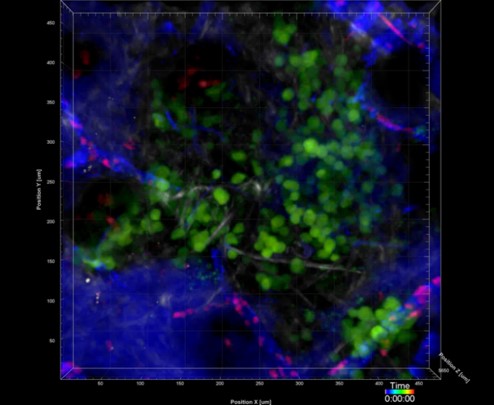
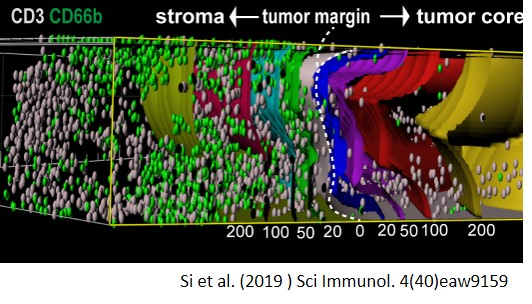
Si Y, Merz SF, Jansen P, Wang B, Bruderek K, Altenhoff P, Mattheis S, Lang S, Gunzer M, Klode J, Squire A, Brandau S, (2019) Multidimensional imaging provides evidence for down-regulation of T cell effector function by MDSC in human cancer tissue. Sci Immunol. 4(40),eaaw9159
https://www.science.org/doi/10.1126/sciimmunol.aaw9159
Lang, Stephan; Bruderek, Kirsten; Kaspar, Cordelia; Höing, Benedikt; Kanaan, Oliver; Dominas, Nina; Hussain, Timon; Droege, Freya; Eyth, Christian; Hadaschik, Boris; Brandau, Sven Clinical Relevance and Suppressive Capacity of Human Myeloid-Derived Suppressor Cell Subset Clinical Cancer Research Vol. 24 (2018) Nr. 19, pp. 4834 – 4844
https://doi.org/10.1158/1078-0432.ccr-17-3726
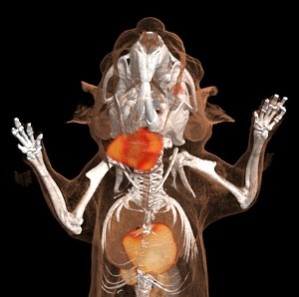
Analysis of neutrophils in the murine tumor host – in vivo veritas
In contrast to the patient-orientated work, which is by nature somewhat descriptive, in this project area we are exploring the in vivo functionality of neutrophils in the tumor host, for example by using high end microscopy, in vivo imaging, and neutrophil manipulating techniques. It is our aim to better understand the in vivo behavior of neutrophils within the tumor host and especially within the tumor microenvironment. To this end, and in collaboration with the Institute for Experimental Immunology, we have performed the “first in mice” studies on tumor-bearing “catchup” mice, which allowed the intravital imaging of genetically traced untouched neutrophils. A second aim in this research theme is to understand how neutrophils modulate primary tumor growth and metastatic spread of tumors. Here we are using syngeneic immunocompetent and xenograft models combined with easily accessible heterotopic or orthotopic localization of the tumors. First data suggest a surprisingly divergent functionality of tumor-associated neutrophils, which is time- and localization-dependent.
Hasenberg A, Hasenberg M, Männ L, Neumann F, Borkenstein L, Stecher M, Kraus A, Engel DR, Klingberg A, Seddigh P, Abdullah Z, Klebow S, Engelmann S, Reinhold A, Brandau S, Seeling M, Waisman A, Schraven B, Göthert JR, Nimmerjahn F, Gunzer M. (2015) Catchup: a mouse model for imaging-based tracking and modulation of neutrophil granulocytes. Nat Methods. 12(5):445-52
https://www.nature.com/articles/nmeth.3322
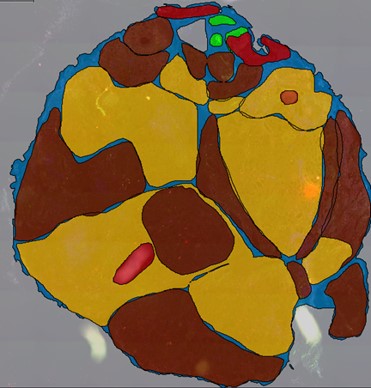
Tumor microenvironment, microbiome and biomarkers
Tumor tissue is not solely an accumulation of proliferating tumor cells, but rather a complex “neo-organ” additionally consisting of mesenchymal-fibroblastoid stromal cells, vasculature, various subsets of immune cells as well as structural matrix components. In this research theme we analyze several aspects of this complex multi-directional interaction. In addition, using well-defined patient cohorts, and in collaboration with the Institute for Pathology, we determine the clinical relevance of selected biomarkers deduced from these studies.
Specifically we explore the following areas:
- Cross-talk between tumor-associated mesenchymal stromal cells and immune cells
- Spatial and functional interaction of immunosuppressive neutrophils and MDSC with tumor-infiltrating T cells
- Impact of bacterial stimulation on the cross-talk of neutrophils and tumor cells
- Impact of the microbiome on the composition and cellular interaction within the tumor microenvironment as well as it´s effect on tumor progression.
- Identification of prognostic and predictive biomarkers in patients with head and neck cancer
Hrvat A, Schmidt M, Wagner B, Zwanziger D, Kimmig R, Volbracht L, Brandau S, Mallmann-Gottschalk N. (2023) Electrolyte imbalance causes suppression of NK and T cell effector function in malignant ascites. J Exp Clin Cancer Res. 2023 Sep 8;42(1):235.
https://doi.org/10.1186/s13046-023-02798-8
Sody S, Uddin M, Grüneboom A, Görgens A, Giebel B, Gunzer M, Brandau S, (2019) Distinct spatio-temporal dynamics of tumor-associated neutrophils in small tumor lesions, Front. Immunol. 10:1419
https://www.frontiersin.org/articles/10.3389/fimmu.2019.01419/full
Szlachetko JA, Hofmann-Vega F, Budeus B, Schröder LJ, Dumitru CA, Schmidt M, Deuss E, Vollmer S, Hanschmann EM, Busch M, Kehrmann J, Lang S, Dünker N, Hussain T, Brandau S. (2025) Tumor cells that resist neutrophil anticancer cytotoxicity acquire a prometastatic and innate immune escape phenotype. Cell Mol Immunol. 2025;22(5):527-540. https://pmc.ncbi.nlm.nih.gov/articles/PMC12041228/
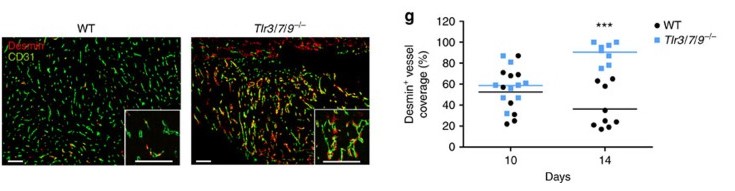
Klein JC, Moses K, Zelinskyy G, Sody S, Buer J, Lang S, Helfrich I, Dittmer U, Kirschning CJ*, Brandau S*. (2017) Combined toll-like receptor 3/7/9 deficiency on host cells results in T-cell-dependent control of tumour growth. Nat Commun. 8:14600. *co-senior authors
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5356072/
Cancer Immunotherapy
Starting several decades ago researchers have been trying to identify means on how to activate and direct the immune system against cancer cells. This has led to several successful therapies, which use either non-antigen-specific adjuvant activities (such as BCG immunotherapy of bladder cancer) or antigen-specific approaches (for example the well-known MAGE antigens). Antibodies, which target tumor cells and/or activate immune cells also made significant contributions. Nevertheless, broad and durable responses have only been achieved in a rather small number of patients. More recently however, the advent of so-called checkpoint blocking antibodies is about to change the field.
Over the last 15 years we have used several immunotherapeutic approaches spanning from bacterial products to TLR agonists or therapeutic antibodies, which were applied to different types of cancer including bladder cancer, lung cancer, ovarian cancer and head and neck cancer. Current projects exploit different antibodies, which either block immunosuppressive pathways in the tumor microenvironment or activate anti-tumor functions of immune cells. In collaboration with the Department of Medical Oncology, we explore immune mechanisms during combinatorial checkpoint inhibitor therapy.
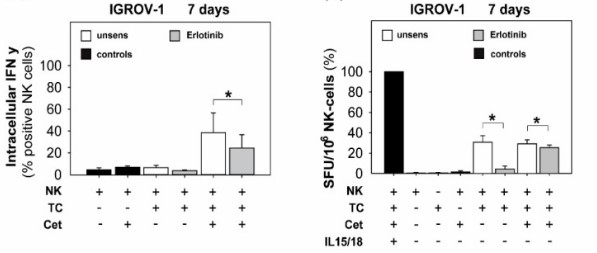
Mallmann-Gottschalk N, Sax Y, Kimmig R, Lang S, Brandau S, (2019) EGFR-Specific Tyrosine Kinase Inhibitor Modifies NK Cell-Mediated Antitumoral Activity against Ovarian Cancer Cells. Int J Mol Sci. 20(19).
https://doi.org/10.3390/ijms20194693
https://www.mdpi.com/1422-0067/20/19/4693
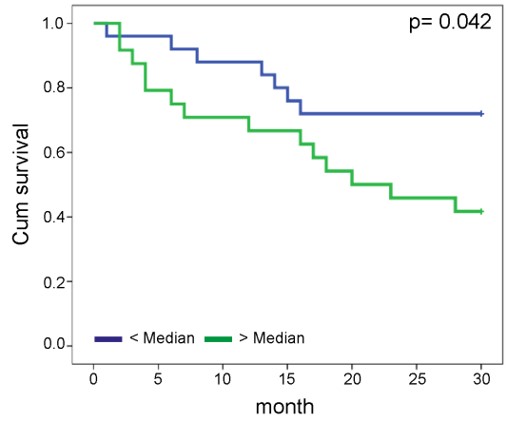
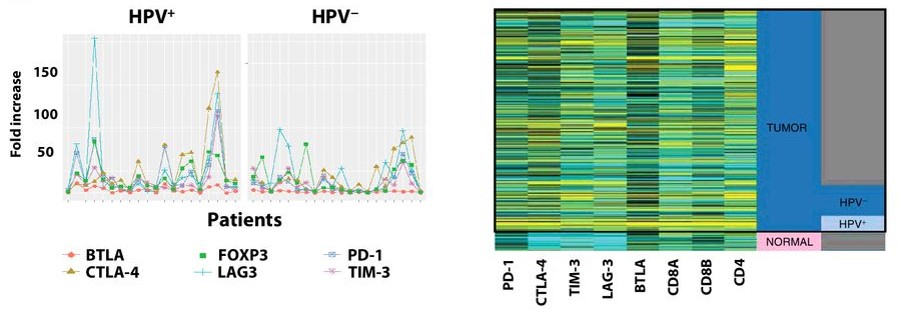
Kansy BA, Concha-Benavente F, Srivastava RM, Jie HB, Shayan G, Lei Y, Moskovitz J, Moy J, Li J, Brandau S, Lang S, Schmitt NC, Freeman GJ, Gooding WE, Clump DA, Ferris RL. (2017) PD-1 Status in CD8(+) T Cells Associates with Survival and Anti-PD-1 Therapeutic Outcomes in Head and Neck Cancer. Cancer Res. 77(22):6353-6364.
DOI: 10.1158/0008-5472.CAN-16-3167
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5690836/
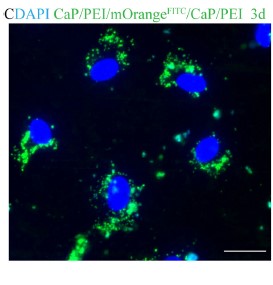
Biomedical application of nanoparticles
Nanoparticles (NPs) are small, microscopic, 3-dimensional particles with a size that does not excedd 100nm. In collaboration with research groups in the Faculty of Chemistry, we are exploring the interaction of NPs with immune cells as well as their potential biomedical applications. NPs can be coupled to antibodies and we are currently testing, whether those constructs can be used to deliver therapeutic compounds to tumor cells or immune cells in order to enhance the anti-tumor activity of such interactions. A second area of major interest for us is the interaction of different classes of NPs with neutrophils as naturally phagocytosing target cells. Although many aspects of the field are in it´s infancy, we are optimistic to accelerate the therapeutic application of NPs by these approaches.
Wey K, Schirrmann R, Diesing D, Lang S, Brandau S, Hansen S, Epple M, (2021) Coating of cochlear implant electrodes with bioactive DNA-loaded calcium phosphate nanoparticles for the local transfection of stimulatory proteins, Biomaterials, 276:121009
https://doi.org/10.1016/j.biomaterials.2021.121009
https://www.sciencedirect.com/science/article/abs/pii/S0142961221003653?via%3Dihub
Schirrmann R, Erkelenz M, Lamers K, Sritharan O, Nachev M, Sures B, Schlücker S, Brandau S.
Gold Nanorods Induce Endoplasmic Reticulum Stress and Autocrine Inflammatory Activation in Human Neutrophils (2022), ACS Nano
doi: 10.1021/acsnano.2c03586.
https://pubs.acs.org/doi/10.1021/acsnano.2c03586
Mesenchymal stromal cells in immunity and oncology
Mesenchymal stromal cells (MSC) are fibroblastoid progenitor cells, which have a distinct multi-lineage differentiation potential. Next to their tissue-regenerative potential MSC have potent immunomodulatory activity, which has led to their further development as cellular therapeutics, primarily in diseases with overshooting immunity. We have explored the interaction of MSC with immune cells and tumor cells in order to better understand their role in immunoregulation and tumor progression. We have also studied the response of MSC to cytokines and TLR ligation as model systems for complex immunological interactions. Mechanistically, we are trying to decipher how MSC use either cell-cell contact, soluble factors or so-called extracellular vesicles (EVs) for their communication with immune and tumor cells. Finally, in collaboration with the Institute for Transfusion Medicine, we have explored means to fine tune the therapeutic activity of MSCs and their EVs by immunological priming.
Hackel A, Aksamit A, Bruderek K, Lang S, Brandau S, (2021)
TNF-alpha and IK-1beta sensitize human MSC for IFN-gamma signaling and enhance neutrophil recruitment.
Eur J Immunol. Eur J Immunol. 51(2):319-330.
https://doi.org/10.1002/eji.201948336
Petri RM, Hackel A, Hahnel K, Dumitru CA, Bruderek K, Flohe SB, Paschen A, Lang S, Brandau S. (2017)
Activated Tissue-Resident Mesenchymal Stromal Cells Regulate Natural Killer Cell Immune and Tissue-Regenerative Function. Stem Cell Reports. 9(3):985-998
https://doi.org/10.1016/j.stemcr.2017.06.020
Team
Head of Research Division

Matthias Schmidt

Kirsten Bruderek
Lab Manager

Dr.
Tanja Hardt-Knechtli

Dr.
Tista Roy Chaudhuri

Dr.
Eva-Maria Hanschmann

Dr.
Benedict Antuamwine

Dr.
Marija Kovacevic Sarmiento

Hendrik Winkel

Francisca Hofmann Vega

Antonio Hrvat
Dr.
Yu Si

Xi Wang

Rachel Giesen

Dr.
Nina Mallmann

PD Dr. med. Anke Daser
Leitende Oberärztin

PD Dr. med.
Benedikt Höing

Dr. med. Cornelius Kürten

Dr. med. Eric Deuß
Selected Publications
Szlachetko JA, Hofmann-Vega F, Budeus B, Schröder LJ, Dumitru CA, Schmidt M, Deuss E, Vollmer S, Hanschmann EM, Busch M, Kehrmann J, Lang S, Dünker N, Hussain T, Brandau S. (2025) Tumor cells that resist neutrophil anticancer cytotoxicity acquire a prometastatic and innate immune escape phenotype. Cell Mol Immunol. 2025;22(5):527-540.
https://pmc.ncbi.nlm.nih.gov/articles/PMC12041228/
Pettinella F, Mariotti B, Lattanzi C, Bruderek K, Donini M, Costa S, Marini O, Iannoto G, Gasperini S, Caveggion E, Castellucci M, Calzetti F, Bianchetto-Aguilera F, Gardiman E, Giani M, Dusi S, Cantini M, Vassanelli A, Pavone D, Milella M, Pilotto S, Biondani P, Höing B, Schleupner MC, Hussain T, Hadaschik B, Kaspar C, Visco C, Tecchio C, Koenderman L, Bazzoni F, Tamassia N, Brandau S, Cassatella MA, Scapini P. (2024) Surface CD52, CD84, and PTGER2 mark mature PMN-MDSCs from cancer patients and G-CSF-treated donors. Cell Rep Med. 2024
https://doi.org/10.1016/j.xcrm.2023.101380
Schirrmann R, Erkelenz M, Lamers K, Sritharan O, Nachev M, Sures B, Schlücker S, Brandau S.
Gold Nanorods Induce Endoplasmic Reticulum Stress and Autocrine Inflammatory Activation in Human Neutrophils (2022), ACS Nano
doi: 10.1021/acsnano.2c03586.
https://pubs.acs.org/doi/10.1021/acsnano.2c03586
Cassetta, Luca; Bruderek, Kirsten; Skrzeczynska-Moncznik, Joanna; Osiecka, Oktawia; Hu, Xiaoying; Rundgren, Ida Marie; Lin, Ang; Santegoets, Kim; Horzum, Utku; Godinho-Santos, Ana; Zelinskyy, Gennadiy; Garcia-Tellez, Thalia; Bjelica, Sunčica; Taciak, Bartłomiej; Kittang, Astrid Olsnes; Höing, Benedikt; Lang, Stephan; Dixon, Michael; Müller, Verena; Utikal, Jochen Sven; Karakoç, Derya; Yilmaz, Kerim Bora; Górka, Emilia; Bodnar, Lubomir; Anastasiou, Olympia Evdoxia; Bourgeois, Christine; Badura, Robert; Kapinska-Mrowiecka, Monika; Gotic, Mirjana; Ter Laan, Mark; Kers-Rebel, Esther; Król, Magdalena; Santibañez, Juan Francisco; Müller-Trutwin, Michaela; Dittmer, Ulf; De Sousa, Ana Espada; Esendaǧli, Güneş; Adema, Gosse; Loré, Karin; Ersvær, Elisabeth; Umansky, Viktor; Pollard, Jeffrey W.; Cichy, Joanna; Brandau, Sven
Differential expansion of circulating human MDSC subsets in patients with cancer, infection and inflammation
In: Journal for ImmunoTherapy of Cancer Vol. 8 (2020) Nr. 2, pp. e001223
Online Full Text: https://doi.org/10.1136/jitc-2020-001223
(Open Access)
Si, Yu; Merz, Simon F.; Jansen, Philipp; Wang, Baoxiao; Bruderek, Kirsten; Altenhoff, Petra; Mattheis, Stefan; Lang, Stephan; Gunzer, Matthias; Klode, Joachim; Squire, Anthony; Brandau, Sven
Multidimensional imaging provides evidence for down-regulation of T cell effector function by MDSC in human cancer tissue
In: Science Immunology Vol. 4 (2019) Nr. 40
Online Full Text: https://doi.org/10.1126/sciimmunol.aaw9159
Lang, Stephan; Bruderek, Kirsten; Kaspar, Cordelia; Höing, Benedikt; Kanaan, Oliver; Dominas, Nina; Hussain, Timon; Droege, Freya; Eyth, Christian; Hadaschik, Boris; Brandau, Sven
Clinical Relevance and Suppressive Capacity of Human Myeloid-Derived Suppressor Cell Subsets
In: Clinical Cancer Research Vol. 24 (2018) Nr. 19, pp. 4834 – 4844
Online Full Text: https://doi.org/10.1158/1078-0432.ccr-17-3726
(Open Access)
Klein, Johanna C.; Moses, Katrin; Zelinskyy, Gennadiy; Sody, Simon; Buer, Jan; Lang, Stephan; Helfrich, Iris; Dittmer, Ulf; Kirschning, Carsten Jürgen; Brandau, Sven
Combined toll-like receptor 3/7/9 deficiency on host cells results in T-cell-dependent control of tumour growth
In: Nature Communications Vol. 8 (2017) pp. 14600
Online Full Text: https://doi.org/10.1038/ncomms14600
(Open Access)
Kansy, Benjamin A.; Concha-Benavente, Fernando; Srivastava, Raghvendra M.; Jie, Hyun-Bae; Shayan, Gulidanna; Lei, Yu; Moskovitz, Jessica; Moy, Jennifer; Li, Jing; Brandau, Sven; Lang, Stephan; Schmitt, Nicole C.; Freeman, Gordon J.; Gooding, William E.; Clump, David A.; Ferris, Robert L.
PD-1 Status in CD8+ T Cells Associates with Survival and Anti-PD-1 Therapeutic Outcomes in Head and Neck Cancer
In: Cancer Research Vol. 77 (2017) Nr. 22, pp. 6353 – 6364
Online Full Text: https://doi.org/10.1158/0008-5472.can-16-3167
(Open Access)
Kalkavan, Halime; Sharma, Piyush; Kasper, Stefan; Helfrich, Iris; Pandyra, Aleksandra A.; Gassa, Asmae; Virchow, Isabel; Flatz, Lukas; Brandenburg, Tim; Namineni, Sukumar; Heikenwalder, Mathias; Höchst, Bastian; Knolle, Percy A.; Wollmann, Guido; Von Laer, Dorothee; Drexler, Ingo; Rathbun, Jessica; Cannon, Paula M.; Scheu, Stefanie; Bauer, Jens; Chauhan, Jagat; Häussinger, Dieter; Willimsky, Gerald; Löhning, Max; Schadendorf, Dirk; Brandau, Sven; Schuler, Martin; Lang, Philipp A.; Lang, Karl S.
Spatiotemporally restricted arenavirus replication induces immune surveillance and type i interferon-dependent tumour regression
In: Nature Communications Vol. 8 (2017) pp. 14447
Online Full Text: https://doi.org/10.1038/ncomms14447
(Open Access)
Bronte, Vincenzo; Brandau, Sven; Chen, Shu-Hsia; Colombo, Mario P; Frey, Alan B; Greten, Tim F; Mandruzzato, Susanna; Murray, Peter J; Ochoa, Augusto; Ostrand-Rosenberg, Suzanne; Rodriguez, Paulo C; Sica, Antonio; Umansky, Viktor; Vonderheide, Robert H; Gabrilovich, Dmitry I
Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards
In: Nature Communications Vol. 7 (2016) pp. 12150
Online Full Text: https://doi.org/10.1038/ncomms12150
(Open Access)
Moses K, Brandau S.
Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells.
In: Semin Immunol. 28 (2) (2016) , 187-96. Online Full Text: https://doi.org/10.1016/j.smim.2016.03.018
Hasenberg, Anja; Hasenberg, Mike; Männ, Linda; Neumann, Franziska; Borkenstein, Lars; Stecher, Manuel; Kraus, Andreas; Engel, Daniel Robert; Klingberg, Anika; Seddigh, Pegah; Abdullah, Zeinab; Klebow, Sabrina; Engelmann, Swen; Reinhold, Annegret; Brandau, Sven; Seeling, Michaela; Waisman, Ari; Schraven, Burkhart; Göthert, Joachim Rudolf; Nimmerjahn, Falk; Gunzer, Matthias
Catchup : a mouse model for imaging-based tracking and modulation of neutrophil granulocytesIn: Nature Methods Vol. 12 (2015) Nr. 5, pp. 445 – 452
Online Full Text: https://doi.org/10.1038/nmeth.3322
Davey MS, Tamassia N, Rossato M, Bazzoni F, Calzetti F, Bruderek K, Sironi M, Zimmer L, Bottazzi B, Mantovani A, Brandau S, Moser B, Eberl M, Cassatella MA
Failure to detect production of IL-10 by activated human neutrophils.
In: Nat Immunol. 12(11) (2011) :1017-8. Online Full Text:
https://doi.org/10.1038/ni.2111
Editors: Sven Brandau, Anca Dorhoi
Myeloid-Derived Suppressor Cells
In: Methods in Molecular Biology.book series
(MIMB, volume 2236)
https://link.springer.com/book/10.1007/978-1-0716-1060-2
Lab Equipment, Methods, Technology
Molecular Biology
- PCR
- Light-Cyler, quantitative PCR
- Basic DNA-, RNA-, protein analytic, Gels, Blots etc.
- Protein arrays
- RNASeq, transcriptomics
Cell Culture/ Cell Biology
- Sterile work bench, cell culture
- Primary cell culture
- Cell separation via MACS
- All essential techniques of cellular immunology
- Antibody arrays
Analytics
- Fluorescence microscope
- Digital Image Analysis and Digital Pathology
- Flow cytometer
- Synergy
- Luminex-Multi-Analyte-Technology
- Microtome/ Immunohistology laboratory
- IMCES: intravital 2-Photon Microscopy,
Light Sheet Microscopy, IVIS, Mouse Ultrasound
Preclinical/ Clinical
- Preclinical murine tumor models
- Anaylysis of patient material
- Investigator initiated trials
Cooperations and Networks
UMESCIA : University Medicine Essen Medical Scientist Academy

Gefördert von:

UMESCIA